When Does Something Become Quantum?
The line between classical and quantum physics is a blurry line, but one general benchmark is if the wavelength of the particle is at least as large as the characteristic size of the system.
Temperature and Wavelength
One of quantum theory’s central concepts is that everything has a wavelength associated with it. The reason why we don’t notice the wave-like properties of everyday objects because their momentum are so massive that it makes the particles’ wave unnoticeable.
Physicist Louis de Broglie proposed a relation between the momentum and wavelength of particles in 1924. He suggested that as the momentum of a particle gets larger, the wavelength gets smaller. Since momentum is a measure of mass and velocity, the wavelength of particles depends on these properties as well.
If you want to bring out the quantum properties of a particle without changing its mass, you can decrease the velocity by cooling it down. The colder something is, the less kinetic energy it has. And the cooler something is, the larger its wavelength is — it becomes “more quantum.” But this depends on the characteristic size of the system, which is the size of the space the particle is bound to.
Cooling Down Copper
In solid copper, as an example, the electron in the outer shell of each atom is loosely bound to the nucleus and shared between atoms within the lattice — so the characteristic distance is the lattice spacing. Since solid copper’s lattice spacing is typically around 3.7 times the diameter of a hydrogen atom, we can calculate the temperature when the valence electron’s de Broglie wavelength becomes as large as the lattice spacing.
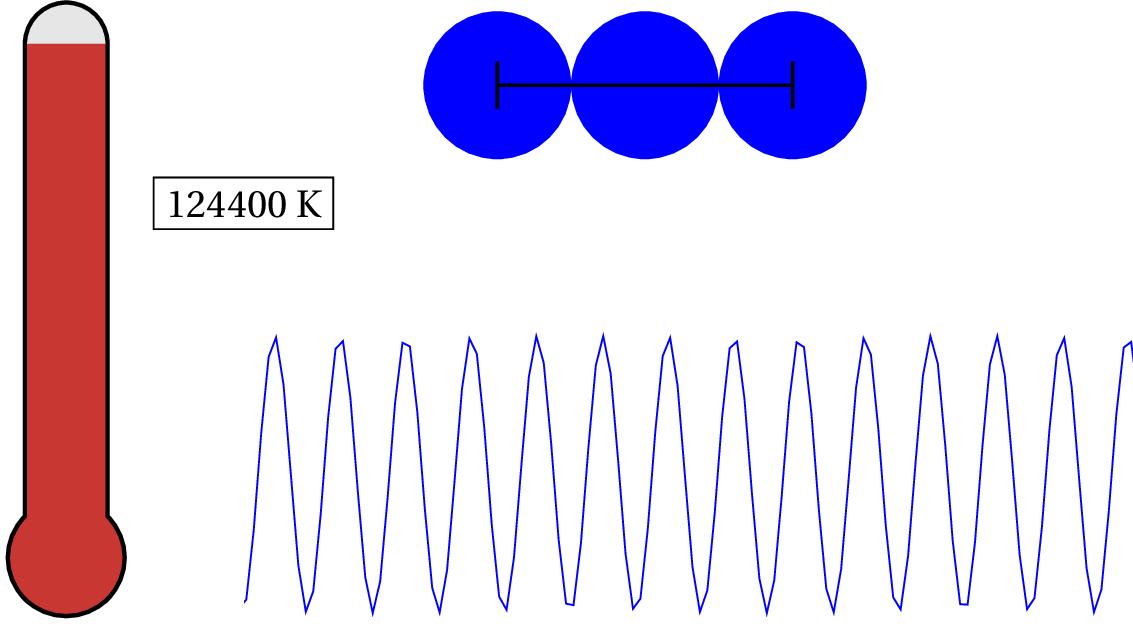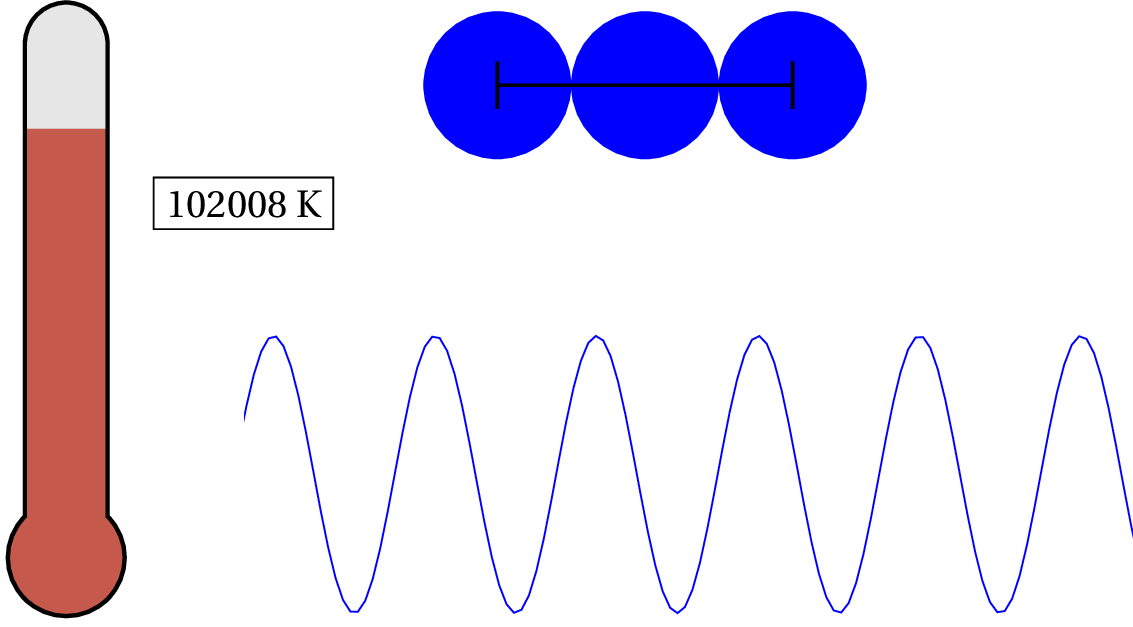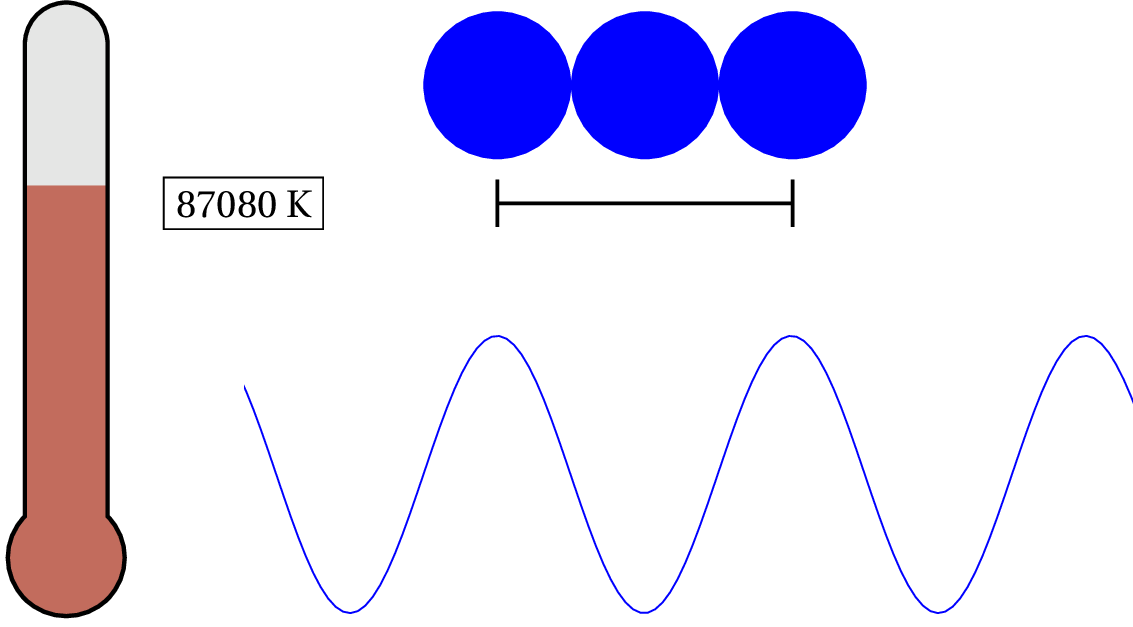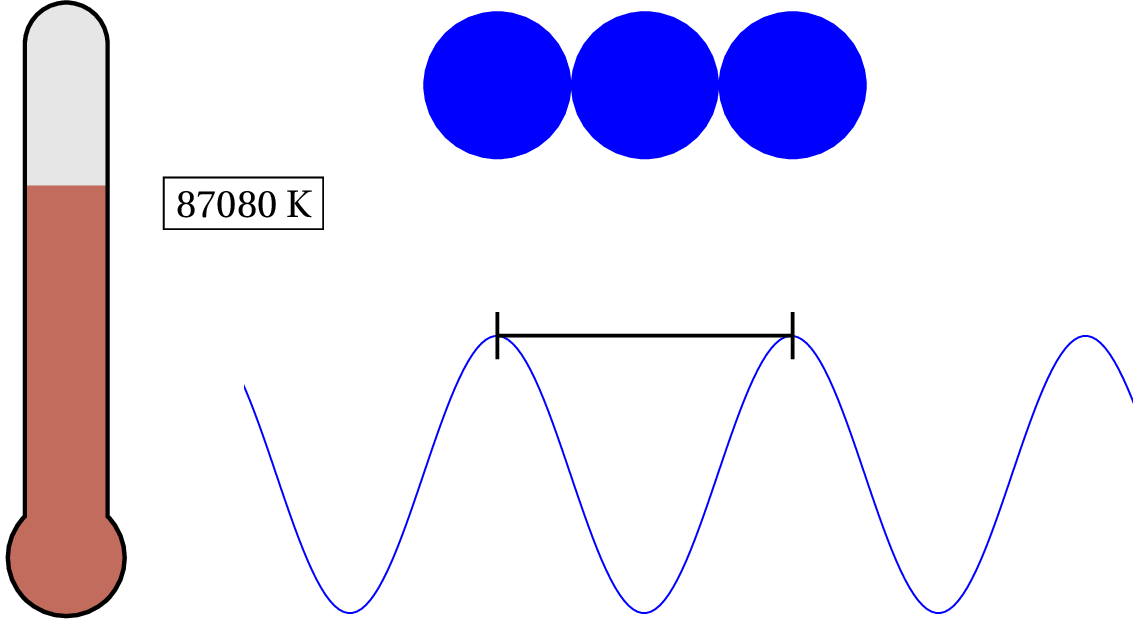
As we lower the temperature of solid copper, the electron is considered to be a quantum object at around 87,075 K — that’s pretty hot! For general purposes, we can consider the valence electron in solid copper (and most metals) as always quantum. But what about the nucleus of the atoms? At what temperature would we have to cool the clump of protons and neutrons to see any quantum effects?
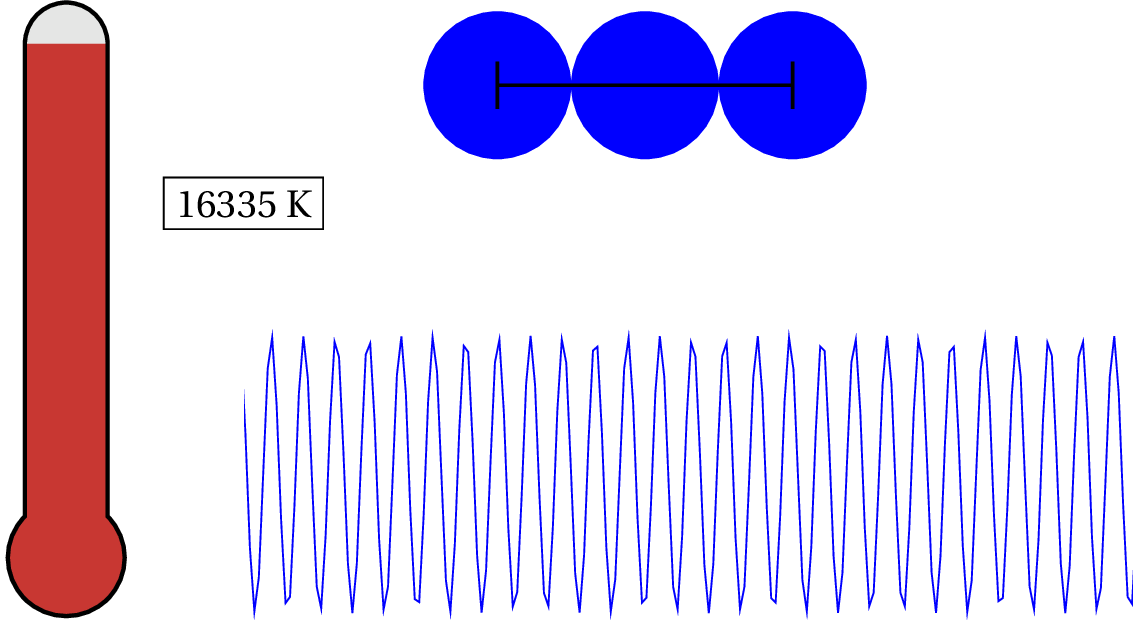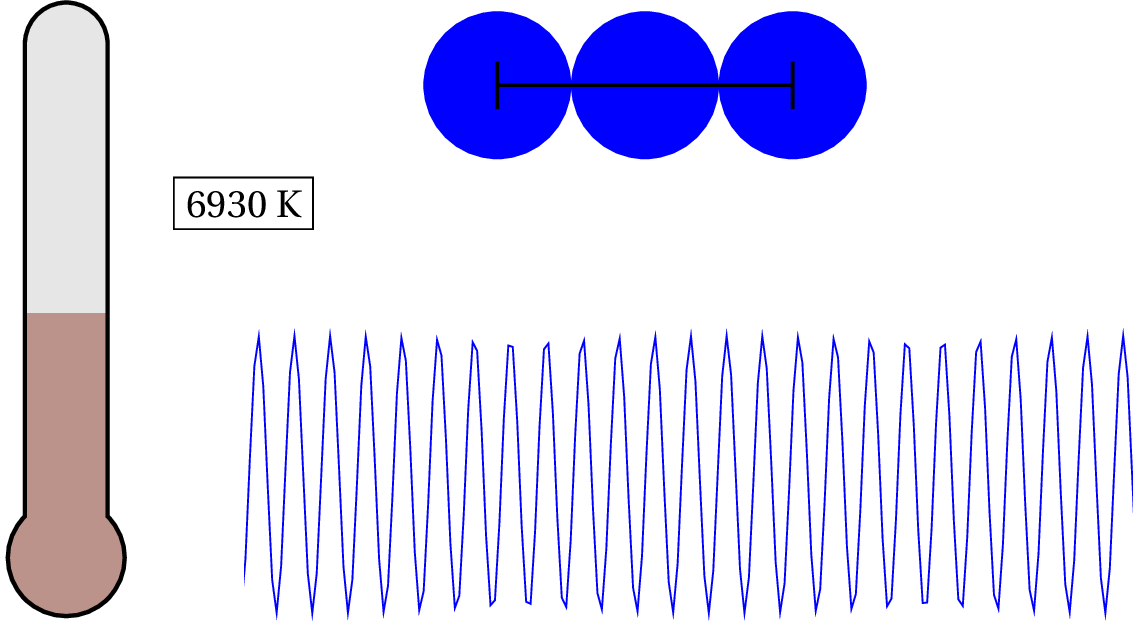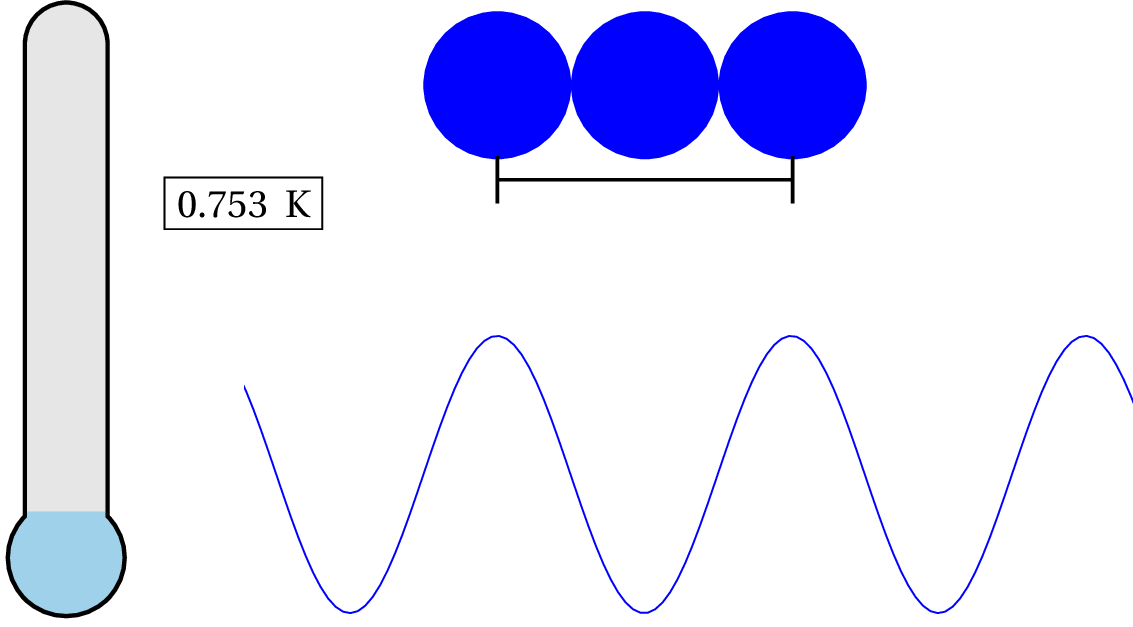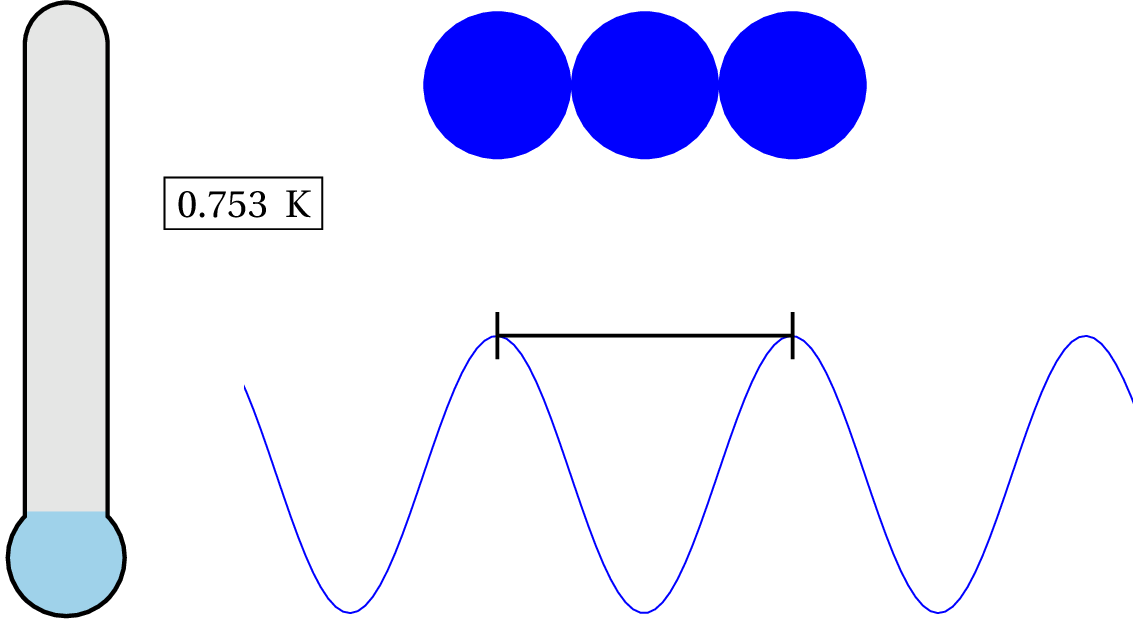
As you can see in the animation, we have to lower the temperature down to .753 K to consider it a quantum system. So unless you cool the system down to nearly absolute zero, then the de Broglie wavelength is still smaller than the characteristic size of the system and, therefore, generally, non-quantum!
Enjoy Reading This Article?
Here are some more articles you might like to read next: